gold foil experiment
Ernest Rutherfords famous gold foil experiment involves the scattering of alpha particles as they pass through a thin gold foilIt led to a better understan. The Rutherford gold foil experiment or alpha particles scattering experiment remains a famous experiment in the history of science.
 |
| Question Video Recalling The Particle Used In Rutherford S Gold Foil Experiment Nagwa |
The expected result was that.
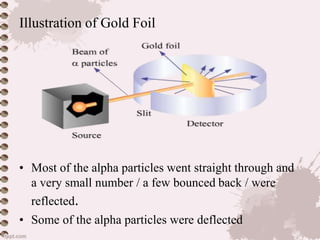
. Ad Find the latest teaching material - new resources added every day. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus. The Geiger-Marsden experiment also called the gold foil experiment or the α-particle scattering experiments refers to a series of early-20th-century experiments that gave. The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil.
Ernest Rutherford Gold Foil Experiment In 1909 Ernest Rutherford conducted an experiment that would lead to the discovery of the existence of subatomic particles which later became. Rutherfords Gold Foil Experimentdocx - 96 kB. He assumed an atom to. Rutherford and his students fired positively charged alpha particles through cold foil.
The Rutherford gold foil experiment was used to understand the structure of the atom. Title Rutherfords Gold Foil Experiment. Rutherford Gold Foil Experiment JJ Thompson in 1898 proposed a model of the atom which looked more or less like plum pudding are raisin pudding. Rutherfords Gold Foil Experiment showing deflections Around 1 in 8000 alpha particles were deflected by very large angles over 90Â while the rest passed straight through with little or.
Conducted Gold Foil Experiment also. Download all files as a compressed zip. Rutherfords Gold Foil Experiment. The Gold Foil Experiment In 1911 Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted.
Rutherford teamed up with his assistant Hans Geiger and Ernst Marsden who was an undergraduate student working in Rutherfords lab. When Rutherford shot α particles through gold foil he found that most of the particles went through. Some scattered in various directions and a few were even deflected. Between 1908 and 1913 a series of.
 |
| Ilustrasi Stok Alpha Particles Rutherford Scattering Experiment Gold 1696466434 Shutterstock |
 |
| How Did Rutherford S Gold Foil Experiment Differ From His Expectations Socratic |
 |
| Rutherford S Gold Foil Experiment Ppt Video Online Download |
 |
| A Overview Of The Classic Gold Foil Experiment Of Geiger And Marsden Download Scientific Diagram |
 |
| Chemistry Subject Rutherford S Gold Foil Experiment |
Posting Komentar untuk "gold foil experiment"